When you feel physically ready and eager to do something, you might describe that feeling as being “full of energy.”
Well, whether it’s a person who feels energy or an object that uses energy, the term “energy” means the power to put something in motion. Energy is something that we use all the time in different ways. Chemical energy is a form of energy that is found in various elements. When we put gasoline in a car, the car uses the stored energy in the gasoline to create physical energy that makes the car perform. When we eat sugar and feel a bit hyper, we are receiving energy from the carbohydrates that are broken down into glucose in our bodies.
But how does chemical energy function in the natural world?
Chemical Energy in Nature
Many chemical processes in the natural world happen in a cycle. You’ve probably heard the expression “circle of life.” When it comes to the use of energy in nature, it’s helpful to think of the processes as occurring in a circular fashion. Plants use solar energy to create sugars from water and carbon dioxide, which allow them to grow. The process causes plants to release oxygen, which we inhale. Our bodies exhale carbon dioxide, which plants use to create sugars, and the process causes them to release more oxygen. The process of plants using solar energy to convert carbon dioxide into sugars is photosynthesis, which is a type of circular use of energy.
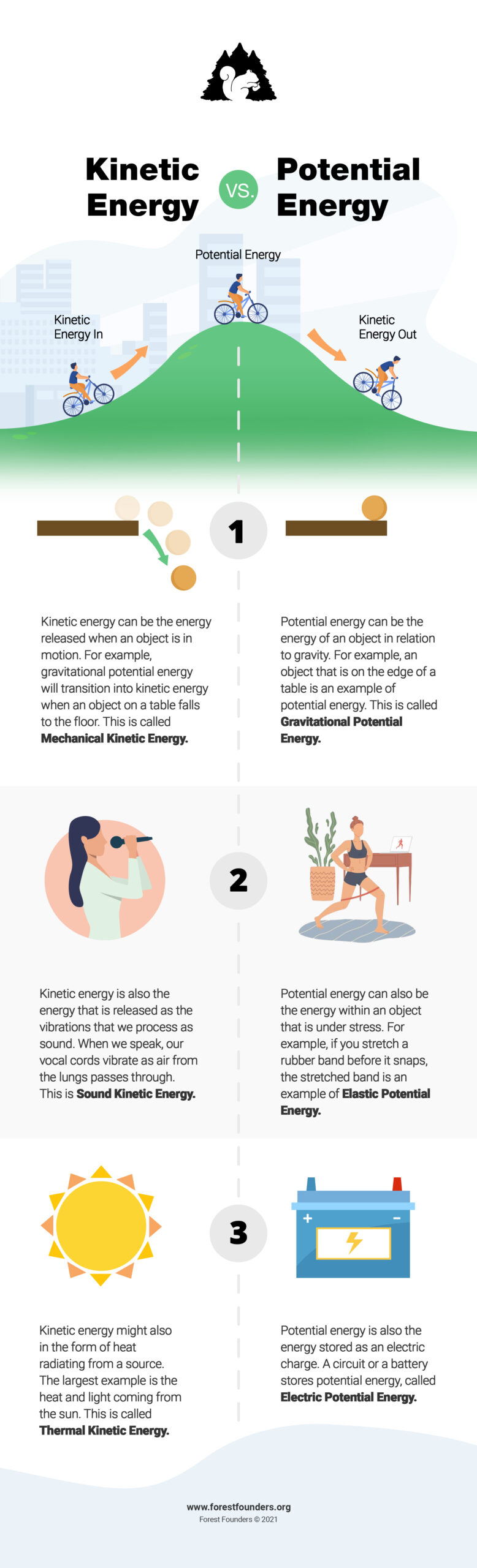
Potential Energy
The chemicals that plants use to grow – carbon dioxide, sugar, and water – are held together by chemical bonds. Chemical bonds are the connections between atoms that create molecules. These bonds are a form of energy, called potential energy. Potential energy is the energy that is held within an object. For example, when a ripe apple is about to fall from an apple tree, the apple’s potential energy is the energy it possesses based upon its distance from the ground and other factors.
The process of photosynthesis is also an example of energy transformation, when one kind of energy is turned into another type of energy. During photosynthesis, energy from the sun is converted into chemical energy in the form of sugars and oxygen.
As we have previously discussed, chemical energy is a form of potential energy that holds the atoms in a chemical together in the form of chemical bonds. But what happens when chemical bonds are broken?
Kinetic Energy
Sugar being digested, either when used in the human body or in plants, is an example of chemical bonds being broken. This process releases energy, which is called kinetic energy. Kinetic energy is the energy released when something is in motion. In the potential energy example above with the apple, the apple’s potential energy is the energy it has when it is about to fall from the apple tree. When the apple falls, the apple’s potential energy is transformed into kinetic energy. In photosynthesis, kinetic energy comes from waves of sunlight, and from the glucose that plants use to build their cell walls. So, what are the processes called when plants use chemical energy?
Exothermic and Endothermic Reactions
When chemicals are broken down so that energy is released, it is called an exothermic reaction. This process releases thermal energy as a result of a chemical reaction. Exothermic reactions release energy in the form of heat, light, electricity, or even sound. A common example is combustion – when you strike a match, the friction causes the potassium chlorate and phosphorus in the match head to ignite and react to the oxygen in the atmosphere, causing a flame. Exothermic means “liberating heat.”
When the opposite occurs – when energy is absorbed so that a chemical reaction can take place – the process is called an endothermic reaction. In photosynthesis, plants need solar energy to convert carbon dioxide into glucose, so the process of photosynthesis is an endothermic reaction. Melting ice is an example of endothermic reactions that you see every day. The absorption of environmental heat causes the bonds within the ice to break, converting the ice into water. Endothermic means “taking in heat.”
The circle of life contains countless examples of naturally occurring chemical processes that contribute to creating the biodiverse world we live in. Unfortunately, many human-influenced chemical processes have led to significant environmental damage that threatens the life and health of every species on Earth. The team at Forest Founders is committed to reversing that environmental damage by restoring and preserving the world’s forests, and supporting tree planting programs that help beautify communities. For more information about Forest Founders’ mission, please visit our information page.